Sol-Gel chemistry offers a flexible approach to obtain a diverse range of materials. It allows different chemistries to be achieved and offers the ability to produce a wide range of nano-structures. Sol-gel materials have been explored and found to hold significant potential. One of the recent developments relates to hybrid materials that utilize sol-gel chemistry to achieve unusual composite properties. Sol-gel chemistry allows the combination of inorganic and organic materials in a single-phase and has led to the development of organic-inorganic hybrid (OIH) coatings for several applications, including corrosion mitigation. The advantages and disadvantages of using modified sol-gel polymer films and hybrid system coatings are discussed, as well as the methodologies for the chemical characterization and the feasibility of evaluating the mechanical properties of the coatings.
Key Words and phrases: Sol-gel methods and chemistry; hybrid, corrosion metallic; polyelectrolyte multilayers; impedance in the layer-by-layer assembly; nanoparticles, polymers, layer-by-layer films; properties of nanoparticles and layer-by-layer polymeric assembly essential in building hybrid coatings; organic precursors in sol-gel methods; mechanism, the binary pore, layer-by-layer films; coatings, capsules.Microstructure and elemental characterization indicate that the finish of the layer-by-layer (LBL) coating consists of a closely connected multilayered coating with a smoother surface. The principles of assembly are also discussed together with the properties of nanoparticles and layer-by-layer polymeric assembly essential in building hybrid coatings.
- Introduction
Aluminum and its alloys are widely used in the fields of engineering, aerospace and machine manufacturing because of their low density, low thermal expansion coefficients, high strength and strain performance, which have drawn much attention from both researchers and engineers as promising materials for electrical appliances and the machine industry. Under certain circumstances, Al alloys possess some anti-corrosion properties because of a naturally formed oxidized thin film. However, the naturally thin and inhomogeneous oxide film substantially dissolves when exposed to corrosive environments containing aggressive chloride ions, resulting in localized corrosion, mechanical failure, considerable financial cost and even catastrophic accidents.
There are two typical approaches to improve the corrosion resistance of Al alloys. One is to ameliorate the element proportion of Al alloys, and the other is to develop a protective film on the Al alloy surface.
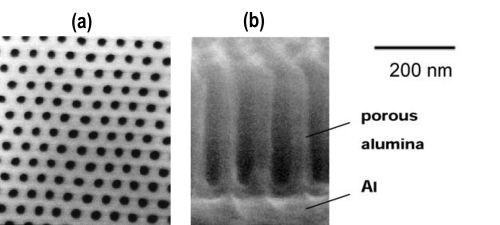
Porous alumina films formed by the anodic oxidation of aluminum have been extensively studied for use as molds to form nanostructured materials. The technology of porous alumina and its usage as an anodic oxide coating in tools has a long history.